Medical Device Software Development: Advancing Healthcare Technology
In today’s rapidly evolving world, technology plays a crucial role in transforming various industries, and healthcare is no exception. Medical device software development has emerged as a vital aspect of modern healthcare, revolutionizing the way medical devices are designed and utilized.
Medical device software development involves the creation of software applications that power medical devices, enabling them to perform complex functions and deliver accurate results. These devices range from diagnostic tools to patient monitoring systems, surgical instruments to drug delivery systems. The integration of software into these devices has significantly improved their functionality, efficiency, and overall patient care.
One of the key advantages of medical device software development is its ability to enhance the accuracy and precision of medical devices. Sophisticated algorithms and machine learning techniques can be incorporated into the software to analyze data collected by the device, allowing for more accurate diagnosis and treatment decisions. This not only improves patient outcomes but also reduces the risk of human error.
Furthermore, medical device software development enables seamless connectivity between devices and healthcare systems. This connectivity allows for real-time data transmission, remote monitoring, and telemedicine applications. Physicians can access patient data from anywhere in the world, enabling timely interventions and personalized care. Additionally, patients can actively participate in their own healthcare by monitoring their vital signs or managing chronic conditions through mobile apps connected to their medical devices.
Safety is a paramount concern in healthcare, and medical device software development addresses this issue effectively. Software developers follow strict regulatory guidelines such as ISO 13485 or IEC 62304 to ensure that medical device software meets stringent quality standards. Rigorous testing procedures are implemented to identify any potential risks or vulnerabilities in the system before it is deployed in clinical settings.
Collaboration between healthcare professionals and software developers is crucial during the development process. Close cooperation ensures that the software meets the specific needs of clinicians and patients while adhering to regulatory requirements. User-friendly interfaces are designed to simplify device operation, reducing the learning curve for healthcare professionals and minimizing the potential for errors.
As technology continues to advance, medical device software development holds immense potential for future innovations. Artificial intelligence, virtual reality, and blockchain technology are already being explored to further enhance medical devices’ capabilities. These advancements have the potential to revolutionize patient care by improving diagnostics, treatment outcomes, and overall healthcare efficiency.
In conclusion, medical device software development has become an integral part of modern healthcare. It has revolutionized the way medical devices are designed, operated, and connected to healthcare systems. Through enhanced accuracy, connectivity, and safety measures, medical device software development is advancing healthcare technology and transforming patient care. As we look towards the future, continued collaboration between healthcare professionals and software developers will drive further innovation in this critical field.
7 Frequently Asked Questions About Medical Device Software Development: A Comprehensive Guide
- What types of software development services are available for medical device software?
- How can medical device software development be tailored to my specific needs?
- What processes and procedures should I follow to ensure successful medical device software development?
- What steps should I take to ensure the safety and efficacy of my medical device software?
- What challenges do I need to consider when developing medical device software?
- How can I ensure that my medical device meets applicable regulatory requirements?
- How do I select the right team for my medical device software project?
What types of software development services are available for medical device software?
When it comes to medical device software development, there are several types of services available to cater to the specific needs of the healthcare industry. These services encompass various stages of software development, from initial concept and design to implementation, testing, and ongoing support. Here are some common types of software development services for medical devices:
- Custom Software Development: This service involves creating tailored software solutions specifically designed for a particular medical device or application. Custom development ensures that the software aligns with the unique requirements and functionalities of the device, maximizing its performance and usability.
- Firmware Development: Firmware refers to the embedded software that controls and operates hardware components within a medical device. Firmware development focuses on creating low-level software that enables seamless communication between hardware components and higher-level applications.
- User Interface (UI) and User Experience (UX) Design: UI/UX design services concentrate on creating intuitive interfaces that facilitate easy interaction between users and medical devices. These services encompass designing visually appealing interfaces with clear navigation, ensuring a smooth user experience.
- Connectivity Solutions: With the rise of connected healthcare systems, connectivity solutions have become crucial in medical device software development. These services involve integrating devices with wireless technologies (such as Bluetooth or Wi-Fi) or implementing secure data transfer protocols to enable seamless communication between devices, healthcare providers, and patient records systems.
- Data Analytics and Machine Learning: Leveraging advanced data analytics techniques and machine learning algorithms can enhance the capabilities of medical devices by enabling real-time data analysis, predictive modeling, or personalized treatment recommendations based on patient data.
- Regulatory Compliance: Medical device software must meet strict regulatory guidelines to ensure patient safety and compliance with industry standards. Regulatory compliance services assist in navigating complex regulations such as FDA (Food and Drug Administration) guidelines in the United States or CE marking requirements in Europe.
- Testing and Quality Assurance: Comprehensive testing is crucial to ensure that medical device software performs reliably under various conditions without compromising patient safety. Testing services include functional testing, performance testing, security testing, and validation to identify and address any potential issues or vulnerabilities.
- Maintenance and Support: Once the software is deployed, ongoing maintenance and support services are essential to ensure smooth operation, address any software-related issues, and provide timely updates or upgrades as needed.
It’s important to note that these services can be provided individually or as part of a comprehensive end-to-end solution, depending on the specific requirements of the medical device project. Collaborating with experienced software development companies specializing in medical devices can help ensure compliance, quality, and successful implementation of innovative solutions in the healthcare industry.
How can medical device software development be tailored to my specific needs?
Medical device software development can be tailored to your specific needs through a collaborative and iterative process. Here are a few key steps to consider:
- Define your requirements: Start by clearly defining your specific needs and goals for the medical device software. Consider factors such as the intended use, target users, desired functionalities, regulatory compliance, and integration with existing systems.
- Engage with a software development team: Find a reputable software development team with experience in medical device software development. Collaborate closely with them to communicate your requirements and ensure they understand your unique needs.
- Conduct thorough analysis: The development team will conduct a thorough analysis of your requirements, including technical feasibility, potential risks, and any regulatory constraints. This analysis will help shape the development approach and identify any potential challenges early on.
- Agile development approach: Adopt an agile development approach that allows for flexibility and iterative feedback loops. This methodology enables you to provide continuous input during the development process, ensuring that the software aligns with your evolving needs.
- User-centered design: Prioritize user-centered design principles to create an intuitive and user-friendly interface. Involve end-users (such as healthcare professionals or patients) in usability testing and gather their feedback to refine the software’s functionality and user experience.
- Regulatory compliance: Ensure that the software development process adheres to relevant regulatory standards (e.g., FDA regulations). Work closely with the development team to ensure proper documentation, risk management processes, validation testing, and adherence to quality standards throughout the project.
- Testing and validation: Rigorous testing is crucial to ensure the safety, reliability, and performance of medical device software. The development team should conduct comprehensive testing at various stages of development to identify any issues or bugs early on.
- Iterative improvements: Once the initial version of the medical device software is developed and deployed, continue gathering user feedback and monitor its performance in real-world settings. Use this feedback to drive iterative improvements and updates to meet evolving needs and address any issues that arise.
Remember, effective communication and collaboration with the software development team are key throughout the process. By tailoring the development process to your specific needs, you can ensure that the resulting medical device software meets your requirements, complies with regulations, and ultimately improves patient care.
What processes and procedures should I follow to ensure successful medical device software development?
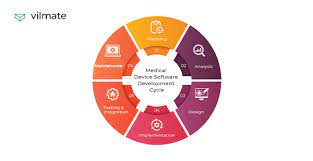
Developing medical device software requires adherence to specific processes and procedures to ensure its successful development and regulatory compliance. Here are some key steps to follow:
- Requirements Gathering: Clearly define the requirements of the medical device software, considering the intended use, functionality, and user needs. Involve stakeholders such as clinicians, patients, regulatory experts, and software developers in this process.
- Risk Analysis: Perform a comprehensive risk analysis to identify potential hazards associated with the software. This includes assessing risks related to patient safety, data security, usability, and system failures. Mitigation strategies should be developed to minimize or eliminate identified risks.
- Software Design: Based on the requirements and risk analysis, create a detailed software design that outlines the system architecture, interfaces, algorithms, data flow, and user interface design. The design should align with relevant regulatory standards such as IEC 62304.
- Implementation: Develop the software code according to the defined design specifications using appropriate programming languages and tools. Follow best practices for coding standards and maintain proper documentation throughout this phase.
- Verification and Validation: Conduct thorough verification activities to ensure that the software meets its intended requirements. This includes unit testing of individual components/modules as well as integration testing of the entire system. Validation activities involve testing the software in simulated or real-world scenarios to demonstrate its safety and performance.
- Documentation: Maintain comprehensive documentation throughout the development process as evidence of compliance with regulatory requirements. This includes documenting design specifications, test plans/results, risk management reports, and any changes made during development.
- Regulatory Compliance: Familiarize yourself with relevant regulations such as FDA’s Quality System Regulation (QSR) or EU’s Medical Device Regulation (MDR). Ensure that your development processes comply with these regulations by conducting regular audits and assessments.
- Usability Testing: Conduct usability tests involving representative users (e.g., clinicians) to evaluate how easily they can interact with the software. Incorporate feedback from these tests to improve the user interface and overall user experience.
- Post-Market Surveillance: Establish a system for monitoring the performance of the software once it is released in the market. This includes collecting and analyzing data related to adverse events, system failures, and user feedback. Implement a mechanism for timely updates and bug fixes based on this surveillance.
- Training and Support: Provide training to users (e.g., healthcare professionals) on how to effectively operate the software and address any questions or concerns they may have. Offer ongoing technical support to ensure smooth usage of the software.
Remember that medical device software development is a complex process that requires expertise in both healthcare and software engineering domains. Engage with experienced professionals, follow industry best practices, and maintain open communication with stakeholders throughout the development lifecycle to ensure successful outcomes.
What steps should I take to ensure the safety and efficacy of my medical device software?
Ensuring the safety and efficacy of medical device software is of utmost importance to protect patients and meet regulatory requirements. Here are some steps you should consider taking:
- Regulatory Compliance: Familiarize yourself with the regulatory guidelines specific to your region, such as FDA regulations in the United States or EU Medical Device Regulations in Europe. Understand the applicable standards, such as ISO 13485 or IEC 62304, and ensure that your software development process complies with these standards.
- Risk Management: Conduct a thorough risk analysis to identify potential hazards and risks associated with your medical device software. Implement risk management practices, such as hazard identification, risk assessment, and risk mitigation strategies. Regularly review and update your risk management plan throughout the development lifecycle.
- Software Development Lifecycle: Follow a well-defined software development lifecycle (SDLC) that includes phases such as requirements gathering, design, implementation, testing, verification, validation, and maintenance. Each phase should have documented processes to ensure traceability and quality control.
- Verification and Validation: Perform rigorous verification and validation activities to ensure that your medical device software meets its intended purpose accurately and safely. Verification involves reviewing documentation, conducting inspections, and performing tests to confirm that the software meets specified requirements. Validation involves testing the software in its intended environment to ensure it performs as expected.
- Usability Engineering: Incorporate usability engineering principles into your software development process to enhance user experience and reduce potential errors or misuse of the device. Conduct usability studies involving healthcare professionals who will be using the software to gather feedback on its design, functionality, and ease of use.
- Quality Assurance: Implement a robust quality assurance program throughout the development process to maintain high-quality standards for your medical device software. This includes establishing quality objectives, conducting internal audits, implementing corrective actions when necessary, and ensuring compliance with relevant quality management systems.
- Documentation: Maintain accurate documentation throughout the entire development process. This includes requirements specifications, design documents, test plans, risk management files, and any other relevant documentation. Documentation should be comprehensive, up-to-date, and easily accessible for audits or regulatory inspections.
- Post-Market Surveillance: Establish a post-market surveillance system to monitor the performance of your medical device software once it is deployed in clinical settings. Collect feedback from users, conduct post-market studies if necessary, and address any reported issues promptly to ensure continuous improvement and patient safety.
- Training and Support: Provide comprehensive training to healthcare professionals who will be using your medical device software. Ensure that they understand its functionality, limitations, and proper usage to minimize the risk of errors or adverse events. Offer ongoing support to address any technical issues or concerns that may arise.
By following these steps, you can enhance the safety and efficacy of your medical device software while meeting regulatory requirements and delivering high-quality products that positively impact patient care.
What challenges do I need to consider when developing medical device software?
Developing medical device software comes with unique challenges that need to be carefully considered to ensure the safety, effectiveness, and compliance of the software. Here are some key challenges to keep in mind:
- Regulatory Compliance: Medical device software development must adhere to strict regulatory guidelines and standards, such as ISO 13485 or IEC 62304. Compliance with regulations like FDA (Food and Drug Administration) requirements is crucial to ensure patient safety and avoid legal issues.
- Risk Management: Assessing and managing risks associated with medical device software is essential. Identifying potential hazards, conducting risk analysis, and implementing risk mitigation strategies are critical steps in the development process.
- Safety and Reliability: Medical devices play a crucial role in patient care, so ensuring the safety and reliability of the software is paramount. Robust testing procedures should be implemented to identify any potential failures or vulnerabilities that could compromise patient health.
- Usability and User Experience: Medical device software should be intuitive, user-friendly, and designed with end-users in mind. Healthcare professionals using the software need to easily navigate through interfaces, interpret data accurately, and perform tasks efficiently.
- Interoperability: In today’s healthcare landscape, interoperability is vital for seamless data exchange between different devices and systems. Ensuring that medical device software can integrate with other healthcare systems or electronic health records (EHRs) is crucial for efficient care coordination.
- Cybersecurity: With the increasing connectivity of medical devices, cybersecurity becomes a significant concern. Protecting sensitive patient data from unauthorized access or cyber threats requires robust security measures such as encryption protocols, secure authentication mechanisms, regular updates, and vulnerability assessments.
- Software Validation: Rigorous testing procedures should be followed to validate the functionality of medical device software throughout its development lifecycle. Verification and validation activities help ensure that the software meets its intended purpose accurately.
- Maintenance and Updates: Medical devices have long lifecycles, so planning for ongoing maintenance, bug fixes, and software updates is essential. Regular updates are necessary to address security vulnerabilities, improve functionality, and comply with changing regulations.
- User Training and Support: Providing comprehensive training and support to healthcare professionals using the software is crucial. Clear documentation, user manuals, and responsive technical support can help users effectively operate the software and troubleshoot any issues that may arise.
- Ethical Considerations: Medical device software development should take into account ethical considerations such as privacy, data protection, informed consent, and the responsible use of artificial intelligence or machine learning algorithms.
By addressing these challenges proactively during the development process, medical device software can meet the highest standards of safety, effectiveness, and usability while improving patient care outcomes.
How can I ensure that my medical device meets applicable regulatory requirements?
Ensuring that your medical device meets applicable regulatory requirements is essential to ensure patient safety and compliance with industry standards. Here are some key steps to help you navigate the regulatory landscape:
- Research and Familiarize Yourself: Gain a thorough understanding of the regulatory requirements specific to your target market. Identify the relevant regulations, guidelines, and standards that apply to your medical device. Common regulatory bodies include the FDA (Food and Drug Administration) in the United States, CE Marking in Europe, and various national health authorities worldwide.
- Engage Regulatory Experts: Seek guidance from regulatory experts or consultants who specialize in medical device regulations. They can provide valuable insights into the specific requirements for your device type and assist you in developing a comprehensive regulatory strategy.
- Conduct a Regulatory Gap Analysis: Perform a gap analysis to assess your device’s current state against applicable regulations. Identify any gaps or areas where improvements are needed to meet the requirements. This analysis will guide you in developing an action plan.
- Develop a Quality Management System (QMS): Implement a robust QMS based on recognized quality standards such as ISO 13485 or FDA’s Quality System Regulation (QSR). A well-designed QMS ensures that your device is manufactured, tested, and documented according to established processes, thereby meeting regulatory requirements.
- Design Controls: Implement design controls throughout the product development lifecycle to ensure that your device meets its intended use, is safe for patients, and performs as expected. This includes conducting risk assessments, establishing design inputs/outputs, verification/validation activities, and maintaining traceability.
- Clinical Evaluation: For certain devices, clinical data may be required to demonstrate safety and efficacy. Conduct clinical evaluations or studies according to applicable guidelines to gather evidence supporting the performance claims of your device.
- Documentation: Maintain comprehensive documentation throughout the development process. This includes design documentation, risk management files, labeling information, manufacturing records, post-market surveillance data, and adverse event reporting.
- Pre-Submission Interactions: Consider engaging in pre-submission interactions with regulatory authorities. Seek their guidance and feedback on your device’s regulatory pathway, clinical data requirements, and any potential concerns they may have.
- Regulatory Submissions: Prepare and submit a well-documented regulatory submission, such as a 510(k) or Premarket Approval (PMA) application to the FDA or a Technical File/Design Dossier for CE Marking in Europe. Follow the specific requirements outlined by the regulatory bodies.
- Post-Market Compliance: Once your device is on the market, establish post-market surveillance systems to monitor its performance, collect user feedback, and address any reported issues promptly. Comply with reporting requirements for adverse events or field safety corrective actions as mandated by regulators.
Remember that regulatory requirements can vary depending on the type of medical device and the target market. Staying informed about evolving regulations and engaging with experts in the field will help ensure that your medical device meets all applicable regulatory requirements.
How do I select the right team for my medical device software project?
Selecting the right team for your medical device software project is crucial to its success. Here are some key factors to consider when choosing a team:
- Expertise and Experience: Look for a team with expertise in both medical device development and software engineering. They should have experience specifically in medical device software development, understanding the regulatory requirements, quality standards, and best practices in the industry. Assess their track record by reviewing their portfolio and client testimonials.
- Regulatory Compliance: Medical device software development is subject to strict regulatory guidelines, such as FDA regulations in the United States or CE marking requirements in Europe. Ensure that the team has experience working within these regulations and can guide you through the compliance process.
- Understanding of Healthcare Domain: A deep understanding of the healthcare industry is crucial for developing effective medical device software. The team should be familiar with healthcare workflows, patient safety considerations, privacy regulations (such as HIPAA), and interoperability standards (such as HL7 or DICOM).
- Collaboration and Communication: Effective collaboration between your team and the software development team is essential for project success. Look for a team that values open communication, actively involves you in decision-making processes, and provides regular progress updates.
- Quality Assurance Processes: Check if the team follows robust quality assurance processes throughout the software development lifecycle. This includes thorough testing procedures, documentation practices, bug tracking systems, and adherence to international quality standards like ISO 13485 or IEC 62304.
- Scalability and Flexibility: Consider whether the team has the capacity to scale up or down based on your project’s needs. Additionally, assess their ability to adapt to changing requirements or incorporate feedback during different stages of development.
- Security Measures: Medical device software deals with sensitive patient data; therefore, security measures are paramount. Ensure that the team follows best practices for data encryption, access control mechanisms, secure communication protocols, and vulnerability testing.
- Post-Development Support: A reliable team will provide ongoing support and maintenance for your medical device software after its deployment. Inquire about their post-development support services, including bug fixes, updates, and troubleshooting.
- Budget and Timeline: Discuss the project budget and timeline with potential teams to ensure alignment. While cost is an important consideration, prioritize quality and expertise over cost alone to avoid compromising the integrity of your project.
- References and Recommendations: Seek references or recommendations from trusted sources within the healthcare or medical device industry. Their insights can provide valuable information about the team’s reputation, reliability, and overall performance.
By considering these factors and conducting thorough evaluations of potential teams, you can select a competent and reliable team for your medical device software project.